VEST
<< Back to all published studies
The Vest Prevention of Early Sudden Death Trial
Purpose
- This randomized controlled trial was designed to study the use of the WCD and its effect on sudden death and total mortality measured at three months following myocardial infarction among patients who have ventricular dysfunction.
Key Results
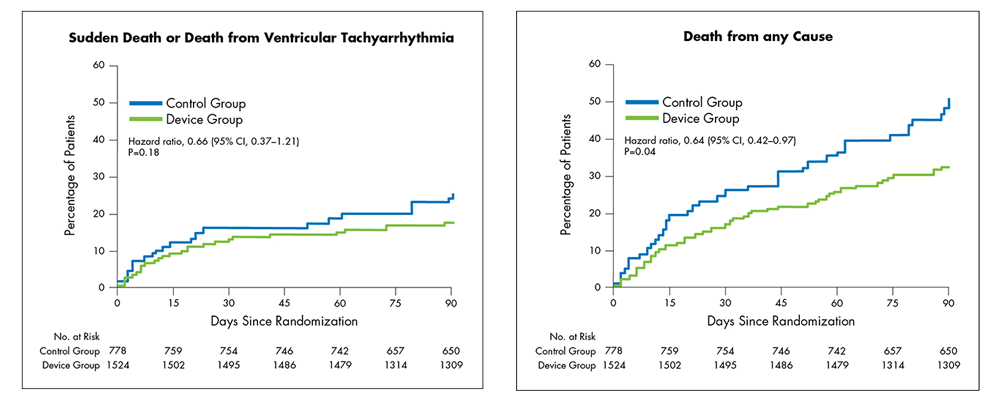
Intent-to-Treat1:
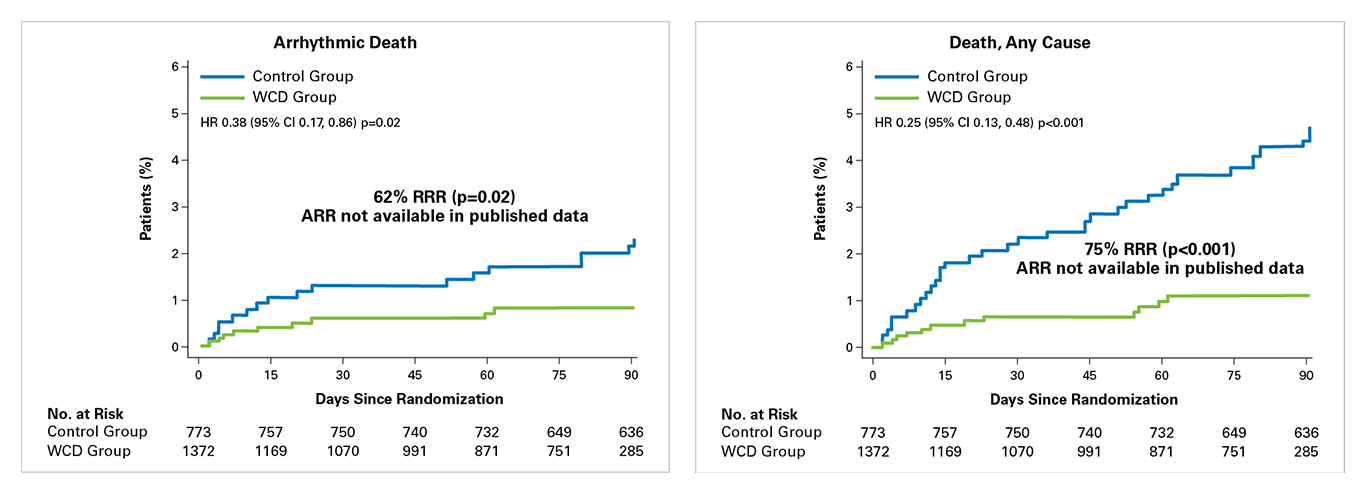
Per-Protocol2:
To evaluate the impact of early stopping of WCD use, investigators performed a per-protocol analysis measuring patient outcomes from randomization to last day of WCD use. Patient data were censored at death, ICD implant, last day the WCD was worn (WCD cohort only; defined as all subsequent days with 0 wear time).
Conclusions
- Mortality is high in the 90 days following an MI.
- 1 in 20 low-EF (EF ≤ 35%) patients died in the 90 days following an MI.
- In an Intent-to-Treat (ITT) analysis1:
- WCD use in post-MI patients with an EF ≤35% resulted in a reduction in the sudden death endpoint, although this did not reach statistical significance at 90 days.
- WCD use resulted in a significant reduction of 36% in total mortality (1.8% ARR; uncorrected p=0.04) at 90 days.
- There was a low rate of inappropriate therapies at <0.6%.
- A per-protocol analysis confirms that when worn, WCD use resulted in significant reductions in sudden death and total mortality2:
- WCD use resulted in a significant reduction of 62% in arrhythmic death (p=0.02).
- WCD use resulted in a significant reduction of 75% in total mortality (p<0.001).
- Among the patients who choose to wear the WCD, median use was high at >22 hours/day.
Background and Methods
- Randomized controlled trial of 2,302 post-MI, EF ≤ 35% patients (mean age of 60.9 ± 12.6 years; mean EF of 28.2% ± 6.1%) randomized 2:1 to receive the WCD plus guideline-directed medical therapy or guideline-directed medical therapy alone.
- Inclusion criteria included post-MI patients with or without revascularization, EF ≤ 35%, and age ≥ 18 years who were enrolled within 7 days of hospital discharge.
- Primary outcome: sudden cardiac death and death due to ventricular arrhythmias at 90 days
- Key secondary outcomes:
- Total mortality
- Non-sudden death (i.e. non-arrhythmic)
- Outcomes were adjudicated through use of hospitalization records, autopsy reports, death certificates and narratives of accounts from witnesses of the events (or circumstances of last being seen). Data from the WCD were not used in the adjudication of outcomes.
- Investigators performed an initial intent-to-treat analysis and subsequent per-protocol analysis.
Click below to read the full publications
1Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N Engl J Med. 2018;379(13):1205–1215.
2Olgin JE, Lee BK, Vittinghoff E, et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J Cardiovasc Electrophysiol 2020;1–10. https://doi.org/10.1111/jce.14404.
<< Back to all published studies
Following a cardiac event, risk of SCD is highest in the first 90 days3,4
3Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–2588.
4Adabag AS, Therneau TM, Gersh BJ, et al. Sudden death after myocardial infarction. JAMA. Circulation 2008;300(17):2022–2029.
Despite PCI & GDMT, 1 in 20 post-MI (EF≤35%) patients die in the first 90 days5
5Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med. 2018;379(13):1205–1215.
SCD accounts for ~50% of mortality following an MI, PCI, or new HF diagnosis (EF≤35%)6,7
6Olgin JE, Pletcher MJ, Vittinghoff E, et al. Wearable cardioverter-defibrillator after myocardial infarction. N Engl J Med. 2018;379(13):1205–1215.
7Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334(21):1349–1355.