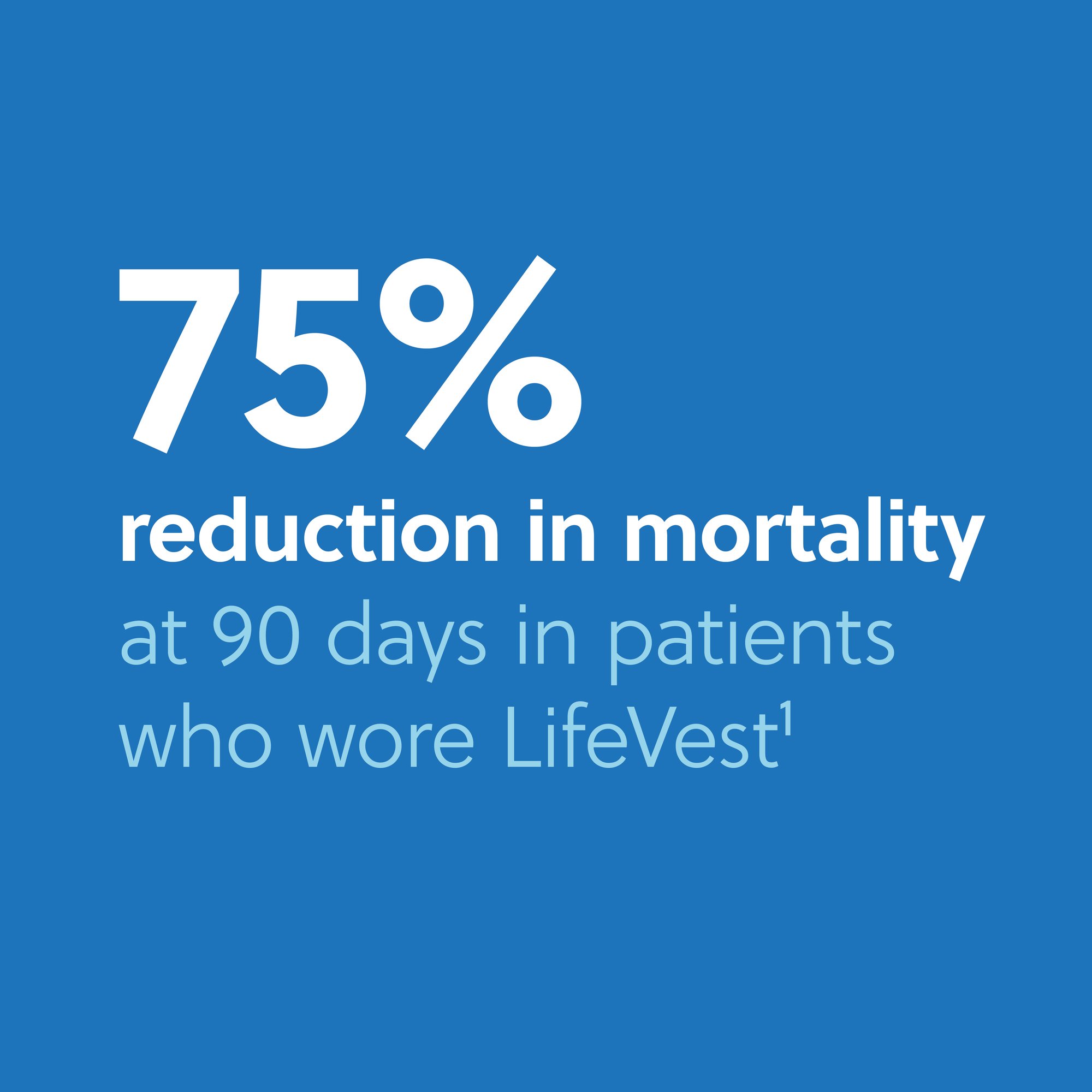

With 20+ years of clinical evidence, LifeVest® is the only WCD proven to reduce mortality in a randomized controlled trial (RCT).





Randomized controlled trial of over 2,000 patients to study the use of the WCD and its effect on sudden death and total mortality in early post-MI, low-EF (EF ≤ 35%) patients at 90 days, including an initial intent-to-treat analysis and subsequent per-protocol analysis. The per-protocol results demonstrated that patients that wore LifeVest realized significant mortality reductions in the first 90 days.


2.6% VT/VF event rate across 11 studies including 19,882 mixed etiology patients (including NICM & ICM)
Meta-analysis of patients prescribed the WCD, which assessed the occurrence of sustained VT events and evaluated the use and effectiveness of WCDs among at-risk cardiac patients.
An AI-enhanced algorithm resulted in zero median false alarms at 90 days in this 96,000 patient analysis
Retrospective analysis of 96,000 commercial LifeVest WCD patients to study the impact of the Advanced Arrhythmia Discrimination Algorithm (AArD), a noise processing algorithm, on the arrhythmia alarm rate.
Long-term survival analysis across more than 3,500 LifeVest WCD patients
Retrospective study compared the long-term survival of more than 3,500 patients who wore the LifeVest WCD to the patients who underwent first ICD implantation.
Safety & efficacy assessment of more than 6,000 LifeVest WCD patients in the German healthcare system
Retrospective study of more than 6,000 German patients evaluated safety and efficacy of the WCD in the German healthcare system.
Assessment of 8,400 patients prescribed the LifeVest WCD during the post-MI waiting period
Retrospective study examined the utility of providing the WCD to more than 8,400 patients determined to be at high risk of SCD during the post-MI waiting period.
90-day survival analysis across nearly 5,000 post-PCI and post-CABG patients prescribed the LifeVest WCD
Retrospective observational parallel cohort study compared mortality outcomes of nearly 5,000 post-revascularization high-risk SCD patients.
ZOLL is ready to assist you in meeting your care goals. The team that has supported more 1M+ LifeVest patients over 20+ years now offers a suite of solutions. Connect with an expert for personalized support.
GET IN TOUCH