WEARIT-II Registry
<< Back to all published studies
Use of the Wearable Cardioverter Defibrillator (WCD) in High-Risk Cardiac Patients: Data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry)
Purpose
- Prospective registry designed to provide data on the safety and efficacy of the WCD in a real world setting.
- Identify the rate of ejection fraction improvement and the need for ICD implantation at the end of WCD use.
Methods
- Prospective registry of 2,000 patients (median age = 62 years, median EF = 25%) prescribed a WCD between August 2011 and February 2014.
- Included patients with ischemic cardiomyopathy (n=805, 40%), non-ischemic cardiomyopathy (n=927, 46%), and congenital/inherited heart disease (n=268, 13.4%).
- Clinical data, arrhythmia events, ICD implantation rate, or improvement in ejection fraction (EF) rate were captured.
Results – Initial Study Results1
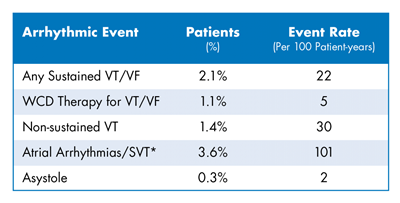
- The sustained VT/VF event rate was high with 22 events per 100 patient-years. 2.1% of patients had a VT/VF event.
- 1 in 14 patients experienced an arrhythmic event.1 The breakdown of these events follows:
- There was a low occurrence of inappropriate therapy (0.5%).
- Patient wear time with the WCD was high with a median daily use of 22.5 hours each day.
- Upon termination of WCD use, EF improved in 41% of patients to the point that an ICD was not longer indicated, while 42% of patients required permanent implantation of an ICD.
Key Results – One-Year Follow-Up Results2
- One-year survival following use of the LifeVest across all patients was high at 96%.
- One-year survival for patients who experienced a VT/VF event during use of the LifeVest was also high at 90%.
Conclusions
- The registry demonstrated a high rate of sustained VT/VF at 3 months in high-risk patients of all etiologies who are not eligible for an ICD, suggesting that a WCD can be safely used to protect patients during this time period of risk assessment.
- In addition to Sudden Cardiac Death (SCD) protection and treatment, the LifeVest WCD captures valuable data about the patient’s broader cardiac function that offers meaningful clinical value during the early period following a cardiac event.
- The high rate of both EF improvement and persistent arrhythmic risk in a high SCD risk population affirms the unpredictability of patient outcomes early after a cardiovascular event.
- Patient prescribed with the LifeVest WCD experience a high one-year survival following LifeVest use.
Click below to read the full publications
1Kutyifa V, Moss AJ, Klein H, et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II Registry). Circulation 2015;132(17):1613–1619.
2Kutyifa V, Moss A, Klein H, et al. One-Year Follow-Up of the Prospective Registry of Patients Using the Wearable Defibrillator (WEARIT-II Registry). Pacing Clin Electrophysiol. 2018;1–7. https://doi.org/10.1111/pace.13448